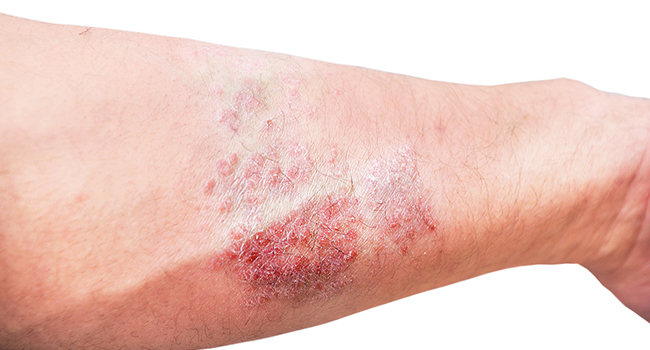
Atopic dermatitis, also known as eczema, is a chronic inflammatory skin condition characterized by red, itchy, and scaly patches of skin. It can range from mild to severe, significantly impacting a person's quality of life, causing intense itching, sleep disturbances, and limiting daily activities. Severe atopic dermatitis can be associated with a higher risk of skin infections and require more intensive and prolonged treatment.
The severity of atopic dermatitis is often assessed by clinical scores such as the Eczema Area and Severity Index (EASI), which considers the extent and severity of the skin lesions, and the Investigator's Global Assessment, which rates the overall severity of the disease.
Clinical Trial
In a clinical trial, researchers investigated the use of rocatinlimab in treating moderate-to-severe atopic dermatitis. The drug targets a protein called OX40, which plays a role in the immune response and the development of T cells. By inhibiting this pathway, the drug is thought to reduce the inflammation associated with atopic dermatitis.The study was a randomized, double-blind, placebo-controlled trial that included 274 adult patients with confirmed atopic dermatitis. Patients were randomized to receive subcutaneous injections of rocatinlimab or placebo every 2 or 4 weeks for up to 18 weeks, followed by an 18-week extension period and a 20-week follow-up. The primary endpoint was the percentage change from baseline in the Eczema Area and Severity Index score at week 16.
The results showed that all doses of rocatinlimab led to significant reductions in EASI scores compared to the placebo at week 16. The most significant decreases were seen in the groups receiving the highest dose of rocatinlimab every two weeks, with a mean reduction in EASI score of 61.1%. These improvements were maintained in most patients after treatment was discontinued.
The drug was generally well tolerated, with the most common adverse events being pyrexia (fever), nasopharyngitis (a common cold), chills, headache, aphthous ulcers, and nausea. There were no deaths reported in the study.
Conclusion
The study suggests that rocatinlimab may be an effective and safe treatment for moderate-to-severe atopic dermatitis. However, further research is needed to confirm these findings and to determine the optimal dose and duration of treatment.__________
ClinicalTrials.gov, Jun-09-22
About Atopic Dermatitis
Breakthrough Treatment for Eczema and Psoriasis
Sitagliptin plus Phototherapy, A Promising Treatment for Psoriasis
Trial finds Deucravacitinib is Effective For Moderate to Severe Plaque Psoriasis
Investigational Drugs bring hope to those suffering from Psoriasis
Netakimab Clinical Trial reveals its efficacy for treating Psoriasis
Can Eczema Medication given to Children cause Cancer?
Are You Concerned About Eczema? See What Doctors Say!
Combining Two Medications Shows Promising Results in Treating Atopic Dermatitis
Managing Pruritus in Atopic Dermatitis with Ruxolitinib Cream
Is Rocatinlimab Safe and Effective for Treating Moderate-to-Severe Atopic Dermatitis?
Drug shows Efficacy against Contact Dermatitis
About Atopic Dermatitis
Breakthrough Treatment for Eczema and Psoriasis
Sitagliptin plus Phototherapy, A Promising Treatment for Psoriasis
Trial finds Deucravacitinib is Effective For Moderate to Severe Plaque Psoriasis
Investigational Drugs bring hope to those suffering from Psoriasis
Netakimab Clinical Trial reveals its efficacy for treating Psoriasis
Can Eczema Medication given to Children cause Cancer?
Are You Concerned About Eczema? See What Doctors Say!
Combining Two Medications Shows Promising Results in Treating Atopic Dermatitis
Managing Pruritus in Atopic Dermatitis with Ruxolitinib Cream
Is Rocatinlimab Safe and Effective for Treating Moderate-to-Severe Atopic Dermatitis?
Drug shows Efficacy against Contact Dermatitis
