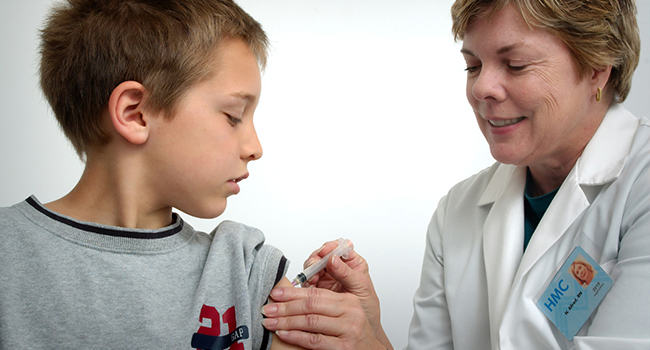
Human Papillomavirus (HPV) is a common sexually transmitted infection that can cause various health issues, including genital warts and cancers of the cervix, vagina, vulva, penis, anus, and throat. The HPV vaccine is a vaccine that can help protect against HPV infection and its related health problems. It is given as a series of shots and is most effective before someone becomes sexually active.
The HPV vaccine is recommended for boys and girls aged 11 or 12. It is also recommended for young adults up to age 26 who did not get vaccinated earlier. The vaccine is most effective when given before someone is exposed to HPV. The HPV vaccine is important because it can help prevent certain types of cancers caused by HPV. It is also important because it can help reduce the spread of HPV.
The HPV vaccine is available at many healthcare providers, such as doctors' offices, clinics, and pharmacies. Insurance usually covers it, and some programs may provide the vaccine for free or at a reduced cost.
Clinical Trial
National organizations have different recommendations on when to start the HPV vaccine, and researchers wanted to understand how these recommendations impact clinical staff's willingness to recommend the vaccine to children. In 2022, researchers surveyed 2,527 clinical staff in the US, including physicians, who provide the HPV vaccine for children. Respondents were randomly assigned to one of three frames based on HPV vaccine recommendations or a no-recommendation control and assessed their willingness to recommend the vaccine for children ages 9-10.
Findings
Recommending HPV vaccination "at ages 11-12" led to a lower willingness to vaccinate at ages 9-10 than the control group. Recommending vaccination "at ages 9-12" led to similar willingness as the control group. However, recommending vaccination "starting at age 9" led to higher willingness than the control group. These results were consistent across respondents' training, specialty, years in practice, clinic's rurality, or healthcare system membership.
Researchers also examined the perceived benefits of recommending HPV vaccination at ages 9 or 12. More common benefits of recommending at age 9 than age 12 were avoiding the topic of sex and completing the vaccine series before age 13. Less common benefits for age 9 were having parents ready to discuss the HPV vaccine and agreeing to vaccination.
Conclusion
It's important to talk to your healthcare provider about the recommended age for HPV vaccination and make informed decisions to protect your health. An effective way to encourage proactive HPV vaccination is to start at age 9. Aligning national recommendations to start at age 9 can promote timely vaccination and help prevent HPV infections and related health problems.
__________

